Mission and
Vision
Francis Medical is a medical device company committed to developing urological cancer treatments that are tough on cancer yet gentle on patients. Our mission is inspired by the belief that minimally invasive interventions hold the potential to halt cancer’s progress. Rooted in our values of quality, integrity, innovation, excellence, and collaboration, we strive to make a meaningful difference in cancer care.
Our
Strategic
Direction
Utilizing the same underlying water vapor technology introduced with Vanquish, Francis Medical plans to broaden its product development scope to include applications targeting bladder and kidney cancers.

In alignment with our commitment to rigorous regulatory standards and patient care, Francis Medical secured U.S. regulatory clearance in December 2025. The company remains dedicated to advancing medical technology responsibly and ethically, with the goal to improve patient outcomes globally.


This breakthrough technology holds the promise of minimizing the life-altering side effects commonly encountered with conventional, more aggressive prostate treatments.
Dr. Christopher Dixon
Chief Medical Officer
Francis’ Story
In 1991, Francis Hoey, Michael’s father, passed away due to prostate cancer complications following treatment that resulted in debilitating side effects. Witnessing firsthand the challenges and limitations of existing treatments, Michael resolved to revolutionize therapeutic approaches in this domain.
Francis Medical was founded based on Michael’s deep expertise in urology and anatomy, passion to find a better solution for men diagnosed with prostate cancer, and love for high-performance race cars.

First Priority: Prostate
Breakthrough Technology
Although this innovative therapy is being designed to address a variety of endourological cancers, initial efforts are focused on the treatment of prostate cancer.
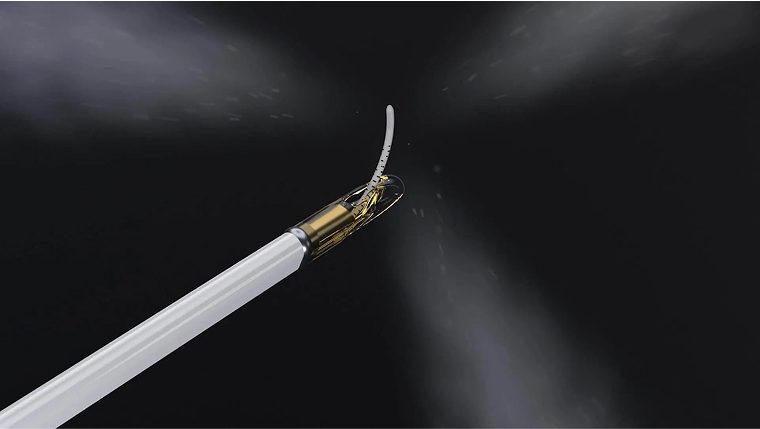
Water vapor technology is designed to treat prostate tissue with the thermal energy stored in sterile water vapor. The Vanquish Water Vapor Ablation System delivers targeted applications of thermal energy using a transurethral procedure.
Traditional prostate treatments include surgery, radiation, hospital stays, and debilitating side effects such as incontinence and sexual dysfunction. In contrast, patients treated with water vapor technology should typically be able to go home the same day and resume their normal lifestyle the very next day.
Timeline: Pioneering the Next Frontier
Water vapor technology is built on ingenuity, passion, physics and decades of combined expertise in urology. Here are some of our top historical milestones.
February 2025
VAPOR 2 pivotal study enrollment is completed two months ahead of schedule. Press Release
January 2025
$80M Series C equity financing is secured to fund the VAPOR 2 study and the U.S. launch of Vanquish for prostate cancer tissue ablation. Press Release
August 2023
FDA grants the Vanquish minimally invasive water vapor ablation therapy Breakthrough Device Designation to expedite its development, assessment, and review so patients have more timelines access. Press Release
July 2023
First patient is treated in the VAPOR 2 pivotal study Press Release
November 2022
Full data from the VAPOR 1 Early Feasibility Study is published in the Journal of Endocrinology. Article
June 2021
Promising six-month primary endpoint results from VAPOR 1 Early Feasibility Study (EFS) are unveiled.
Press Release
September 2020
Coloplast is added to roster of investors. The additional funding allowed the company to optimize its product design and execute the U.S. pivotal study. Press Release
December 2018
Francis Medical launches with $18M Series A funding for promising cancer ablation technology using water vapor. Press Release
May 2018
Francis Medical is formed to explore the potential of water vapor therapy for urological cancers – initially prostate cancer – followed by bladder and kidney cancer.
May 2018
Boston Scientific acquires Rezum to solidify its position in the treatment of BPH and other non-cancerous urological conditions.
August 2015
NxThera achieves FDA clearance for Rezum, an in-office treatment for BPH that uses controlled doses of thermal energy stored in water vapor to reduce prostate gland tissue causing urinary tract symptoms.
June 2008
NxThera is founded by Michael Hoey to pioneer advancements in urology through its innovative water vapor platform technology.
1991
Francis Hoey passes away from prostate cancer. Michael Hoey resolves to revolutionize therapeutic approaches to address the debilitating side effects that occur with current treatments.
- February 2025
- January 2025
- August 2023
- July 2023
- November 2022
- June 2021
- September 2020
- December 2018
- May 2018
- May 2018
- August 2015
- June 2008
- 1991
Our
Leadership.
The Francis Medical leadership and board members use their extensive experience to inspire and guide the team to develop solutions that transform patient lives.
We’re Here
To Help.
Francis Medical is moving rapidly and we would love to connect. Follow us on social media or complete the form to ask a question or be added to our company communications.


 Refer to the device User Manual for a list of contraindications, warnings, and cautions.
Refer to the device User Manual for a list of contraindications, warnings, and cautions. Training: Do not operate the Vanquish System without completing Francis Medical-provided physician training. Untrained operation of the device may lead to improper use. Improper use can result in patient injury or equipment malfunction.
Training: Do not operate the Vanquish System without completing Francis Medical-provided physician training. Untrained operation of the device may lead to improper use. Improper use can result in patient injury or equipment malfunction.