Stay
Up-To-Date
Articles, publications, podcasts, and more to keep you current on our Vanquish Water Vapor Ablation System.
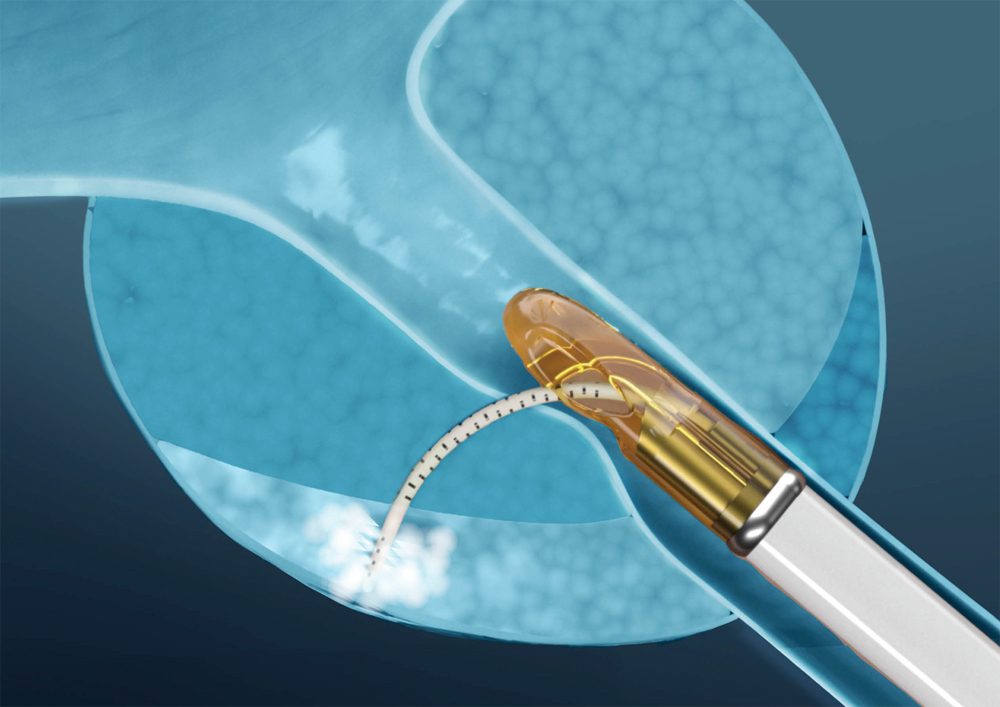
Francis Medical Announces First Commercial Procedure with the Vanquish® Water Vapor System for Prostate Tissue Ablation
MINNEAPOLIS (Feb 3, 2026) – Francis Medical, Inc., a privately-held medical device company, announced the first commercial procedure using the Vanquish Water Vapor Ablation System...
Learn More >

Francis Medical Receives FDA 510(k) Clearance for Use of the Vanquish® Water Vapor System for Prostate Tissue Ablation
MINNEAPOLIS (Dec. 2, 2025) – Francis Medical, Inc.,a privately-held medical device company developing the breakthrough Vanquish® Water Vapor Ablation System ...
Learn More >

Press Release: Francis Medical Announces Completion of Enrollment in the VAPOR 2 Pivotal Study for Vanquish Prostate Cancer Treatment
February 25, 2025: Francis Medical completes enrollment in the company's VAPOR 2 Pivotal Study for Vanquish Prostate Cancer Treatment...
Learn More >

Press Release: Francis Medical Announces Close of Record $80 Million Series C Equity Financing
January 8, 2025: Francis Medical raises record $80M in Series C to fund its VAPOR 2 study and U.S. launch of Vanquish, its proprietary prostate cancer treatment...
Learn More >
CEO Mike Kujak Interviewed by Medtech Insight
In a revealing interview with Citeline Commercial's Medtech Insight, CEO Mike Kujak discusses how Francis Medical's Vanquish system is set to redefine prostate cancer treatment...
Learn More >

The MedTech Startup Podcast: CEO Mike Kujak
Francis Medical CEO Mike Kujak shares the highs and lows of being the CEO of a medtech startup and provides a window into the journey at Francis Medical...
Learn More >
Follow Us
We’re Here
To Help.
We’d love to connect. Follow us on social media or complete the form to ask a question or to be added to our company communications.


 Refer to the device User Manual for a list of contraindications, warnings, and cautions.
Refer to the device User Manual for a list of contraindications, warnings, and cautions. Training: Do not operate the Vanquish System without completing Francis Medical-provided physician training. Untrained operation of the device may lead to improper use. Improper use can result in patient injury or equipment malfunction.
Training: Do not operate the Vanquish System without completing Francis Medical-provided physician training. Untrained operation of the device may lead to improper use. Improper use can result in patient injury or equipment malfunction.