News Article,Video
Maple Grove Medical Device Company Pioneers New Treatment For Prostate Cancer
June 18, 2020

CCX Media visited Francis Medical, Wednesday, June 17, 2020 for exclusive interviews with CEO and President, Mike Kujak, and Founder and CTO, Michael Hoey.
Their two-minute video includes an explanation of our revolutionary water vapor therapy, as well as plans for the future, including our VAPOR 1 clinical study.
Recent Posts
Francis Medical Announces First Commercial Vanquish® Procedures in Arizona for Prostate Tissue Ablation
websites2026-03-04T16:43:25-06:00March 5th, 2026|
MINNEAPOLIS (March 5, 2026) – Francis Medical, Inc., a privately held medical device company, announced today the successful completion of the first commercial (non-clinical trial) procedures in Arizona using the Vanquish® Water Vapor Ablation System for prostate tissue ablation.
Francis Medical Announces First Commercial Procedure with the Vanquish® Water Vapor System for Prostate Tissue Ablation
FMADMIN2026-01-30T13:43:37-06:00February 3rd, 2026|
MINNEAPOLIS (Feb 3, 2026) – Francis Medical, Inc., a privately-held medical device company, announced the first commercial procedure using the Vanquish Water Vapor Ablation System
Francis Medical Receives FDA 510(k) Clearance for Use of the Vanquish® Water Vapor System for Prostate Tissue Ablation
websites2026-01-02T14:35:48-06:00December 2nd, 2025|
MINNEAPOLIS (Dec. 2, 2025) – Francis Medical, Inc.,a privately-held medical device company developing the breakthrough Vanquish® Water Vapor Ablation System


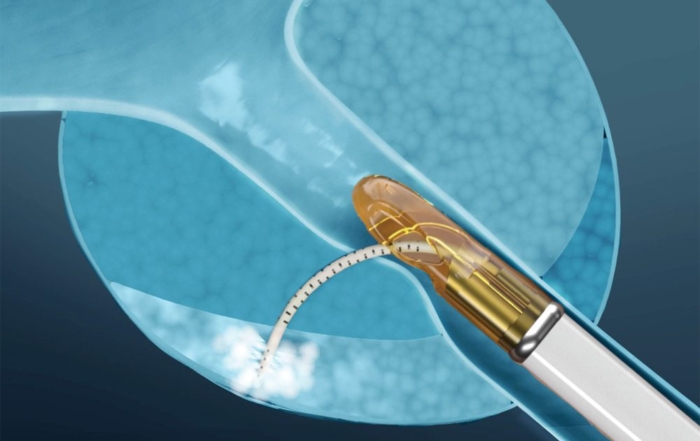


 Refer to the device User Manual for a list of contraindications, warnings, and cautions.
Refer to the device User Manual for a list of contraindications, warnings, and cautions. Training: Do not operate the Vanquish System without completing Francis Medical-provided physician training. Untrained operation of the device may lead to improper use. Improper use can result in patient injury or equipment malfunction.
Training: Do not operate the Vanquish System without completing Francis Medical-provided physician training. Untrained operation of the device may lead to improper use. Improper use can result in patient injury or equipment malfunction.