News Article
Francis Medical Featured in BioWorld MedTech
December 17, 2018

Francis Medical, Inc. has been featured in BioWorld MedTech, a leading news source of advances in medical technology.
Recent Posts
Francis Medical Announces First Commercial Procedure with the Vanquish® Water Vapor System for Prostate Tissue Ablation
FMADMIN2026-01-30T13:43:37-06:00February 3rd, 2026|
MINNEAPOLIS (Feb 3, 2026) – Francis Medical, Inc., a privately-held medical device company, announced the first commercial procedure using the Vanquish Water Vapor Ablation System
Francis Medical Receives FDA 510(k) Clearance for Use of the Vanquish® Water Vapor System for Prostate Tissue Ablation
websites2026-01-02T14:35:48-06:00December 2nd, 2025|
MINNEAPOLIS (Dec. 2, 2025) – Francis Medical, Inc.,a privately-held medical device company developing the breakthrough Vanquish® Water Vapor Ablation System
Press Release: Francis Medical Announces Completion of Enrollment in the VAPOR 2 Pivotal Study for Vanquish Prostate Cancer Treatment
Website Manager2026-01-07T11:41:38-06:00February 25th, 2025|
February 25, 2025: Francis Medical completes enrollment in the company's VAPOR 2 Pivotal Study for Vanquish Prostate Cancer Treatment

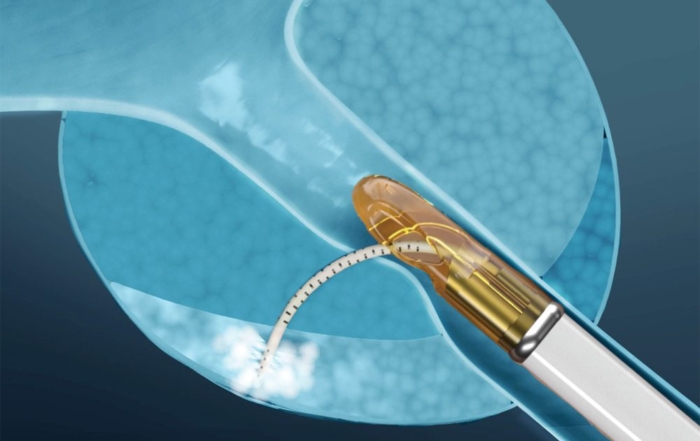



 Refer to the device User Manual for a list of contraindications, warnings, and cautions.
Refer to the device User Manual for a list of contraindications, warnings, and cautions. Training: Do not operate the Vanquish System without completing Francis Medical-provided physician training. Untrained operation of the device may lead to improper use. Improper use can result in patient injury or equipment malfunction.
Training: Do not operate the Vanquish System without completing Francis Medical-provided physician training. Untrained operation of the device may lead to improper use. Improper use can result in patient injury or equipment malfunction.