Francis Medical is dedicated to acquiring clinical data to understand the use of water vapor ablation to manage prostate cancer. Early results are promising.
Clinical
Study Overview
We continue to build upon our VAPOR 1 and VAPOR 2 clinical studies by partnering with experts in prostate cancer to study Vanquish. Check back for publications and information on new studies.
Multi-Center Pivotal Study (n=235)
VAPOR 2 is a novel, multi-center trial using a transurethral thermal water vapor device (Vanquish) to manage localized intermediate-risk prostate cancer. Enrollment is complete and the 235 patients will be followed for 5 years.
Data published on the first 110 patients with 12-month follow-up demonstrated that:
- 91% clearance of targeted MRI visible ≥ Grade Group 2 (GG2) disease following a single treatment
- Ability to treat anywhere in the prostate
- No device-related serious adverse events
93% of patients extremely satisfied or satisfied with the Vanquish procedure
- 2.7% ≥ Grade 2 urinary incontinence
- Low rates of erectile dysfunction
Patients enrolled in the study will have follow-up appointments every 6 months through 5 years.
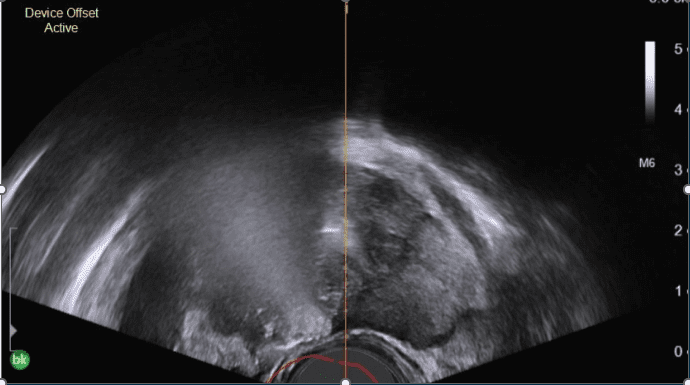
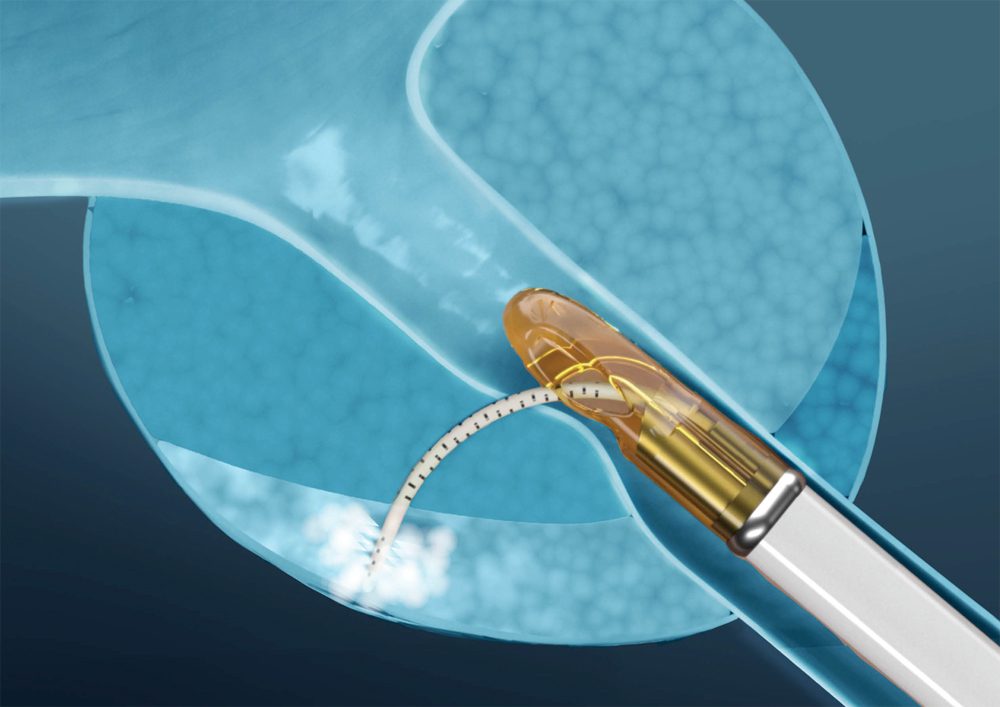
Vapor 1 Early Feasibility Study (n=15)
Vapor 1 studied the feasibility of treating prostate cancer with water vapor at 4 U.S. sites. Hemiablation was performed…
- No serious device-related adverse events reported
- No device-related events reported
- 13/15 (87%) of patients were biopsy-negative for ≥ Gleason pattern 4 disease (Grade Group 2) on the treated side at 6 months
The study demonstrated that water vapor ablation has a low morbidity and it is possible to successfully re-treat residual disease or treat new lesions identified on surveillance, if necessary.

Publications
Purpose: We report results of a prospective, multicenter single-arm study of transurethral vapor ablation (TUVA) of prostate tissue in patients with unilateral, intermediate-risk, localized prostate cancer (PCa).
Warlick CA, Spilseth BD, Dixon CM
Purpose: Targeted and precise application of thermal energy stored in sterile water vapor is a novel approach to treat cancerous prostate tissue. We report safety and oncological results of transurethral hemigland vapor ablation in a prospective, single-arm study in men with unilateral, intermediate-risk localized prostate cancer.
Christopher M. Dixon, Richard M. Levin, Christopher H. Cantrill, Mikhail Regelman, Benjamin Spilseth, Ronald F. Tutrone Jr., Michael A. White, Aaron J. Milbank, Christopher A. Warlick
We’re Here
To Help.
We are dedicated to collecting clinical data on our technology. We will continue to update our website with new publications and studies related to Vanquish. Please reach out with questions.


 Refer to the device User Manual for a list of contraindications, warnings, and cautions.
Refer to the device User Manual for a list of contraindications, warnings, and cautions. Training: Do not operate the Vanquish System without completing Francis Medical-provided physician training. Untrained operation of the device may lead to improper use. Improper use can result in patient injury or equipment malfunction.
Training: Do not operate the Vanquish System without completing Francis Medical-provided physician training. Untrained operation of the device may lead to improper use. Improper use can result in patient injury or equipment malfunction.